
Package Design – FDA Compliance
Today’s topic focuses on basic FDA Compliance tips to help you instill trust and safety in your brand for your customers.
Keep these 10 FDA compliance regulations in mind when designing your package:
1 – Nutrition Facts: The FDA requires the inclusion of a standardized “Nutrition Facts” panel on most packaged food products. This panel provides information about serving sizes, calories, nutrients, and other essential nutritional details.
This is by no means a complete list of regulations – so you should definitely seek guidance from a professional.
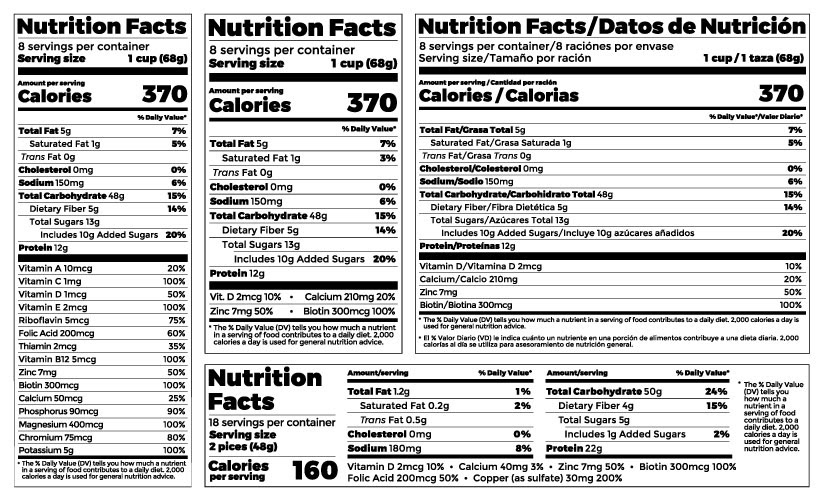
Pro Tip: There are several acceptable layouts for the Facts Panel that are based on the overall size of your package. Consult with a professional to make sure you are using the correct layout for your specific package dimensions.
2 – Ingredient List: The FDA mandates that the packaging includes a detailed list of all ingredients used in the product, starting with the most prominent ingredient and descending in order of quantity.
3 – Allergen Labeling:Manufacturers must clearly identify common food allergens if they are present in the product. These allergens include milk, eggs, fish, shellfish, tree nuts, peanuts, wheat, and soybeans (and most recently, sesame).

4 – FDA Disclaimer: Some products may require specific FDA disclaimers, such as those related to dietary supplements or health claims. These disclaimers must be accurate and compliant with FDA guidelines.
5 – Health Claims: There are clearly defined rules when it comes to the types of claims you can make about a food product’s effect on the consumer’s health. The easiest way to guarantee the wrath of the FDA and a swift food recall is to make an unauthorized health or drug claim. This means making a claim about how the product or one of its ingredients affects your body or provides some sort of therapeutic effect.
Pro Tip: It is not only unlawful to make a health claim on the product label itself, but also on marketing materials (e.g. the company website), even if you have scientific evidence to back it up.
6 – Expiration Date or Best Before Date: Products with limited shelf life must have an expiration date or a “Best Before” date to ensure consumer safety and product quality.
7 – Country of Origin Labeling (COOL): Certain food products may require labeling to indicate the country of origin.
8 – Net Quantity of Contents: The package must accurately state the net weight or volume of the product, typically expressed in ounces, pounds, or milliliters.
Pro Tip: Net Weight statements must be a certain size font and placed away from any interfering graphics so they can be read easily by consumers.
9 – FDA Facility Registration: If you are a food manufacturer or distributor, you must register your facility with the FDA.
10 – Identity Statement: The packaging should clearly display the common or usual name of the product. It should be easily identifiable by consumers.
Pro Tip: FDA labeling requirements clearly require most packaged foods to declare what the product is, usually referred to as the “common name”. This is why a Tostito’s label must clarify that the product is Tortilla Chips and Nutella must describe itself as Hazelnut Spread.
It’s crucial to keep yourself updated on any changes or additions to FDA regulations that may affect your product packaging. Note that the FDA’s regulations apply specifically to the United States. If you plan to export your food product to other countries, you will need to comply with their respective regulatory authorities’ requirements.
It’s crucial to keep yourself updated on any changes or additions to FDA regulations that may affect your product packaging. Note that the FDA’s regulations apply specifically to the United States. If you plan to export your food product to other countries, you will need to comply with their respective regulatory authorities’ requirements.
Final Pro Tip: Consult with an FDA consultant before you go to print with your packaging!